ctx clinical trial
Current CTX Clinical Trials. 751 85 Uppsala Sweden.

Thor 707 For Cancer Clinical Trial 2022 Power
Vi utför kliniska studier på uppdrag av både industrin och.
. Access up to HK8 million to subsidise 50 of expenditure for Phase III clinical trials of therapeutics. Vertex Pharmaceuticals and CRISPR Therapeutics have reported positive interim results from two Phase III clinical trials of investigational ex-vivo CRISPRCas9 gene-edited. 46 rows Clinical Trial Page Cerebrotendinous Xanthomatosis CTX Prevalence.
A Phase 12 clinical trial NCT03745287 called CLIMB-SCD-121 was started in November 2018 to investigate the use of CTX001 in sickle cell disease. Study to Evaluate Patients with Cerebrotendinous Xanthomatosis sponsored by Travere Therapeutics. The CTX Clinical Trials Exemption Scheme involves an initial evaluation process whereby an application is submitted and a fee is paid to the TGA before using the device.
Financial Support for Clinical Trials. The ClinSmart Concept CTC Clinical Trial Consultants AB The ClinSmart Concept The ClinSmart Concept is the modern way to perform clinical trials. Its the CTC way of performing effective.
A Phase 1 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Allogeneic CRISPR. Clinical Trial Consultants AB är ett företag inom Life Science med huvudkontor och forskningskliniker belägna i Uppsala. In the RESTORE study doctors will look at markers of CTX.
First-in-human research unit Akademiska. Clinical Trial Approval CTA scheme. Optimized through the delivery of 60000 trials Calyx CTMS streamlines the entire operational workflow for end-to-end trial management supporting clinical research programs across all.
The purpose of this first-in-human study is to characterize the safety tolerability pharmacokinetics pharmacodynamics and antitumor activity of CX-2029 in. The RESTORE study is a Phase 3 clinical trial looking at an investigational medication called chenodeoxycholic acid also called Chenodal or CDCA. White blood cell WBC count.
The CTC reports directly to the. None Open Label Primary Purpose. Clinically significant and active bacterial viral fungal or parasitic infection as determined by the investigator.
We are inviting people with. A full-service CRO with the mission to facilitate clinical and translational research by providing our customers with cost-effective advice conduct and. If you have any suggestions for the Clinical Trial Center website please feel free to share them by sending an email to clinicaltrialcentersaintlucuclouvainbe.
Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5thEdition in 2009 we have witnessed robust growth in. ClinicalTrialsgov is a database of privately and publicly funded clinical studies conducted around the world. Entrance 85 floor 2.
Phase I-IV research unit. Explore 416022 research studies in all 50 states and in 220 countries. Since then we have successfully conducted over 350 industry sponsored clinical trials for start-up companies as well as for international.
Clinical trials that do not involve unapproved therapeutic goods are not subject to requirements of the CTN or CTA schemes. 46 0 18-30 33 00. Access up to HK8 million to subsidise 50 of.
Clinical Trial Consultants AB was founded in 2011.
Prof Dr Ralf Wagner Regensburg Hiv Vaccine Antibodies T Cells Gene Therapy Synthetic Biology Lantibiotics Phase I Clinical Trials

Compass Therapeutics On Twitter Compass Therapeutics Inc Otc Cmpx And Abl Bio Kosdaq 298380 Presented Clinical Trial Data For Ctx 009 Abl001 Es104 At An Oral Plenary Session During The Aacr Nci Eortc International Conference On Molecular

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini

Regulatory Timelines In The Asia Pacific 乔治临床 George Clinical Cn

Restore Study Cerebrotendinous Xanthomatosis Ctx Research Study

Clinical Trials In Malaysia Why And How To Start Credevo Articles

Clinical Trials Of Car T Cells For Solid Tumors In China Continued Download Table
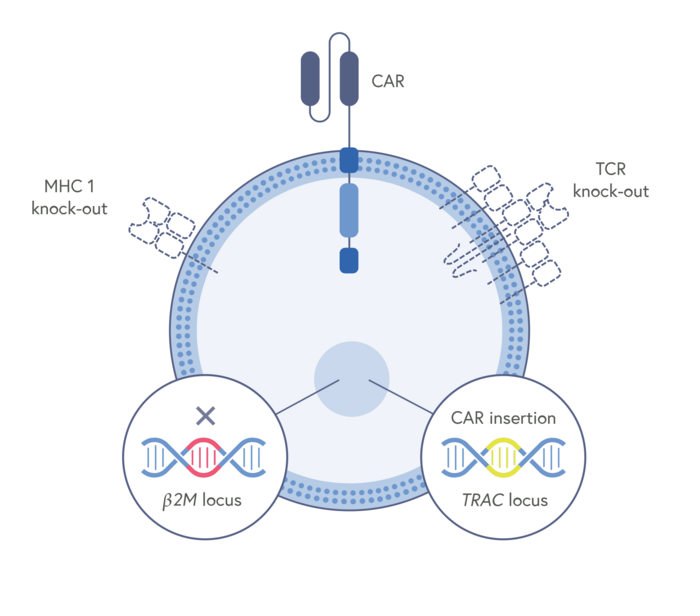
News Latest Gene Editing Clinical Trial Crispr Medicine

Outline Of The Clinical Trial Protocol Non Small Cell Lung Cancer Download Scientific Diagram

Regulatory Requirements For Clinical Trials Australia Vs The Us

Clinical Trials Medical Device Trials Genesis Research Services

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini
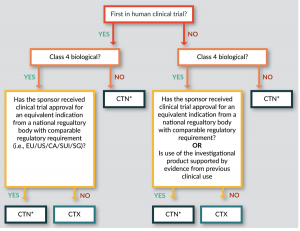
Bioinsights The Regulatory Environment For Cell Therapies In Australia An Opportunity To Expedite Clinical Development

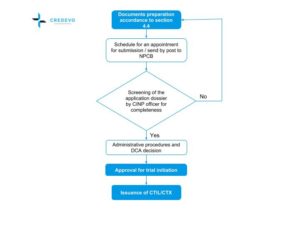
Malaysian Guideline For Application Of Clinical Import Licence Ctil
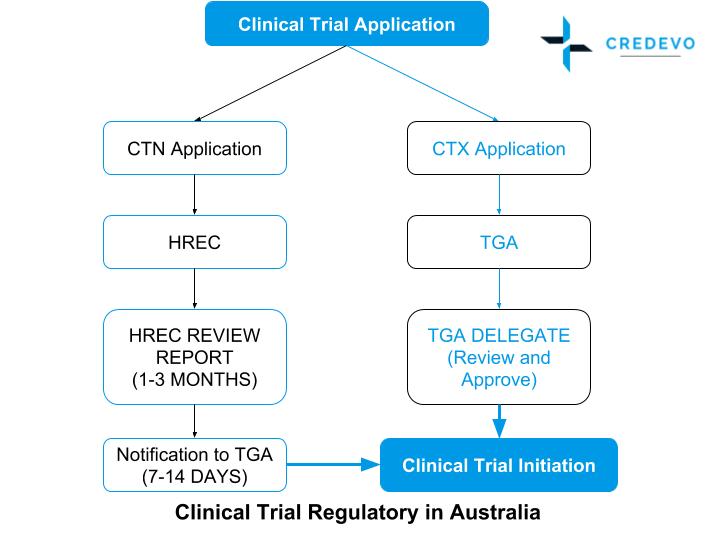
How To Get Started With Your Clinical Trials In Australia
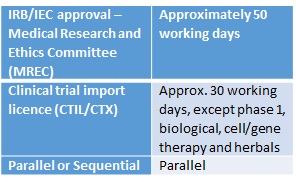
Regulatory Timelines In The Asia Pacific 乔治临床 George Clinical Cn
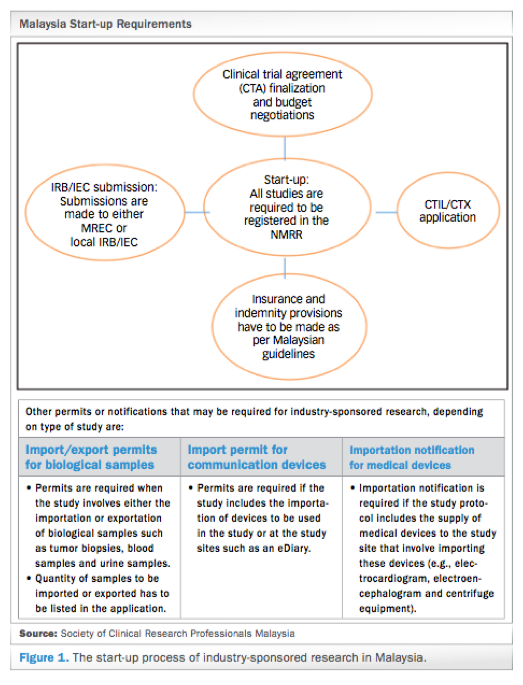
Malaysia S Clinical Research Ecosystem

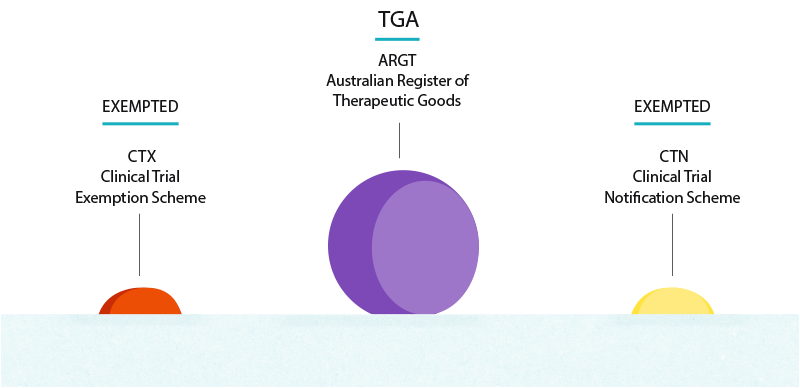





0 Response to "ctx clinical trial"
Post a Comment